Prioritised medicines for MPP licensing
By expanding access to key medicines, MPP is committed to supporting Universal Health Coverage
![]()
Prioritisation of medicines ensures that MPP focuses its efforts on interventions for which such mechanism could have the greatest public health impact.

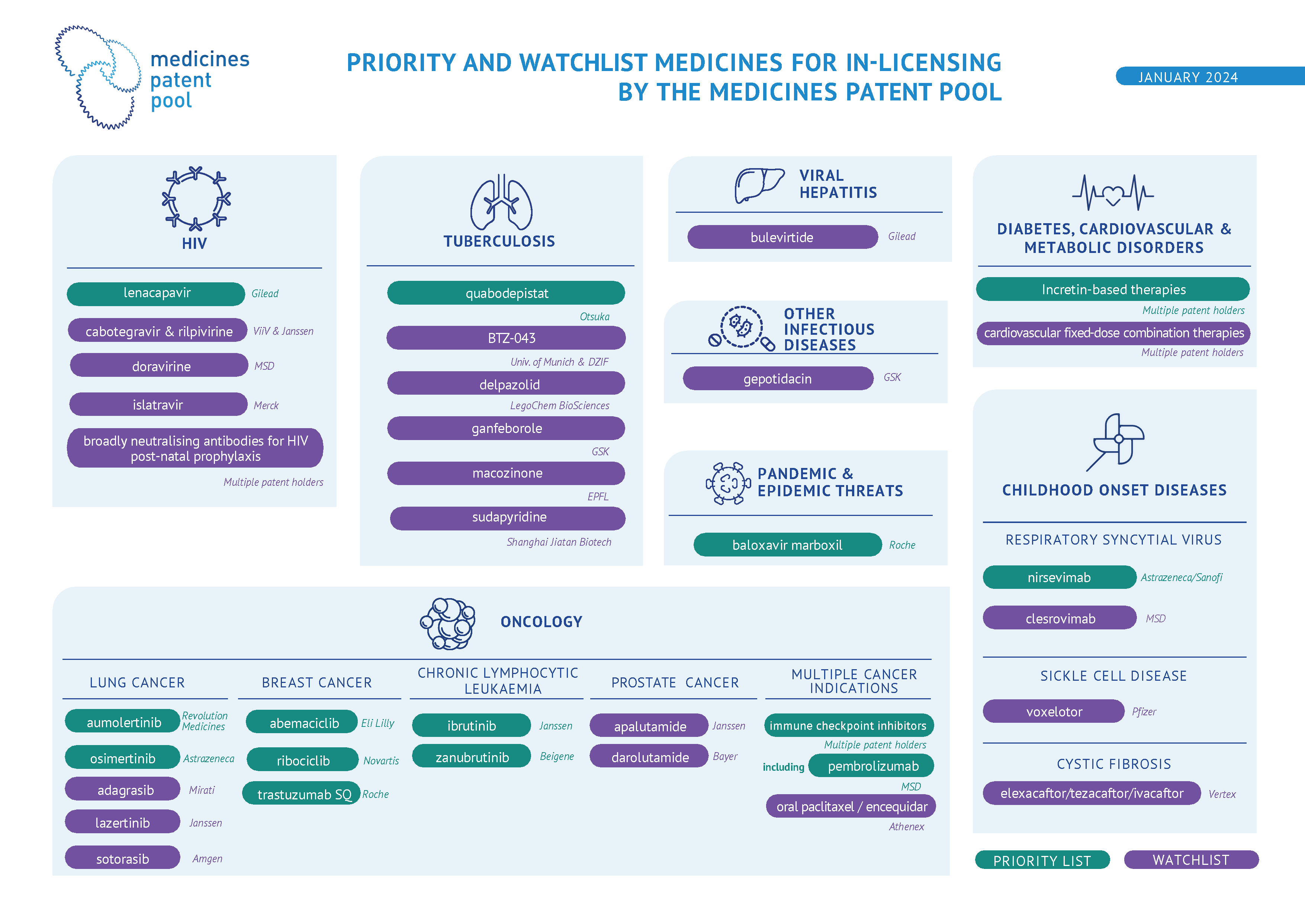
HIV

VIRAL HEPATITIS

TUBERCULOSIS

PANDEMIC & EPIDEMIC THREATS
INFLUENZA
OTHER INFECTIOUS DISEASES

DIABETES, CARDIOVASCULAR & METABOLIC DISORDERS
DIABETES
CARDIOVASCULAR & METABOLIC DISORDERS

MATERNAL HEALTH

CHILDHOOD ONSET DISEASES
CYSTIC FIBROSIS
RESPIRATORY SYNCYTIAL VIRUS
SICKLE CELL DISEASE

ONCOLOGY
MULTIPLE CANCER INDICATIONS
LUNG CANCER
BREAST CANCER
CHRONIC LYMPHOCYTIC LEUKAEMIA
PROSTATE CANCER
There are 39 million people living with HIV worldwide, but only 29.8 million currently receive antiretroviral therapy. HIV paediatric care is improving, but still only one in two children living with the virus has access to treatment1. IV prevention is also key to tackle the transmission of the disease. Although Pre-Exposure Prophylaxis (PrEP) has proven efficient in preventing HIV infection, its uptake is still slow.
Affordable, effective HIV medicines are imperative, especially for people living with HIV in low- and middle-income countries (LMICs) where HIV is most prevalent. Medicines must also be available in the right formulations. Fixed-dose combinations increase adherence. Special treatments for children, appropriate for different ages and weights, improve care.
Since 2010, we have worked with leading HIV drug manufacturers, governments, international organisations, civil society, and affected communities2 to improve access to World Health Organization prioritised and recommended medicines for people living with HIV in LMICs. We have also worked to increase access to HIV prevention tools and support the diversification of prevention options. In 2022, MPP signed a voluntary licensing agreement with ViiV Healthcare for cabotegravir long-acting (LA) for HIV pre-exposure prophylaxis (PrEP). This is an important step in accelerating affordable and equitable access to long-acting PrEP in over 90 countries3.
1 UNAIDS: Global HIV & AIDS statistics — 2021 fact sheet (last accessed on 21 Sept. 2023).
2 In the framework of MPP’s work with communities, MPP relies on its Community Advisory Panel, a group of experts with lived experience that support MPP in the implantation of our activities.
3 MPP is collaboratively working with the LA PrEP Coalition.
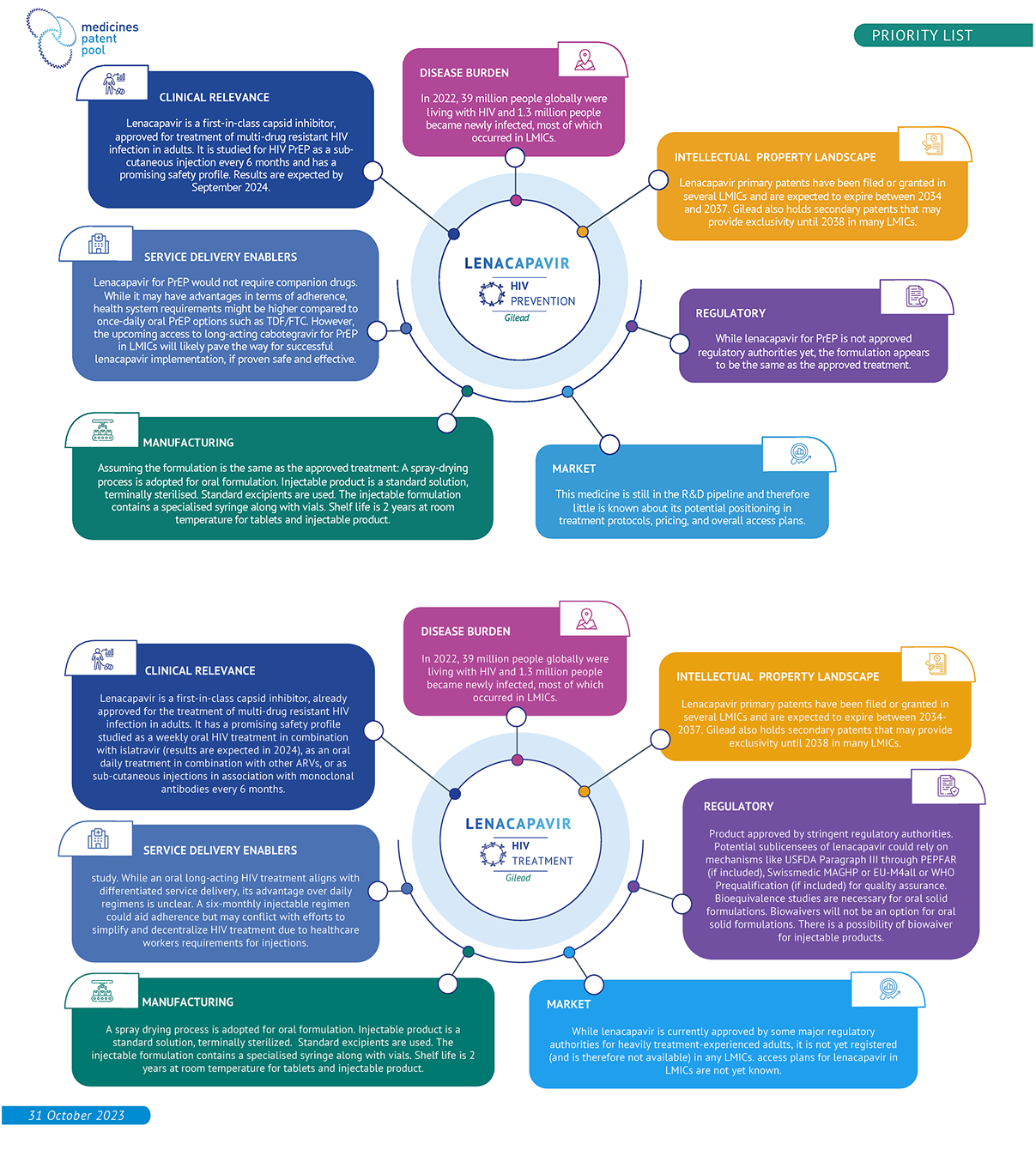
Lenacapavir is an MPP priority since 2022. Its new mechanism of action and its long-acting properties (up to 6 months dosing interval) will likely make it an important product in HIV prevention and/or treatment (in combination with other medicines). The clinical programs continue to indicate favourable efficacy and safety profiles. Furthermore, lenacapavir has been prioritised by WHO in both PADO-5 and CADO-41.
Patent & licence data in LMICs
1 Priorities for antiretroviral drug optimization in adults and children report of a CADO, PADO and HIVResNet joint meeting (accessible here).
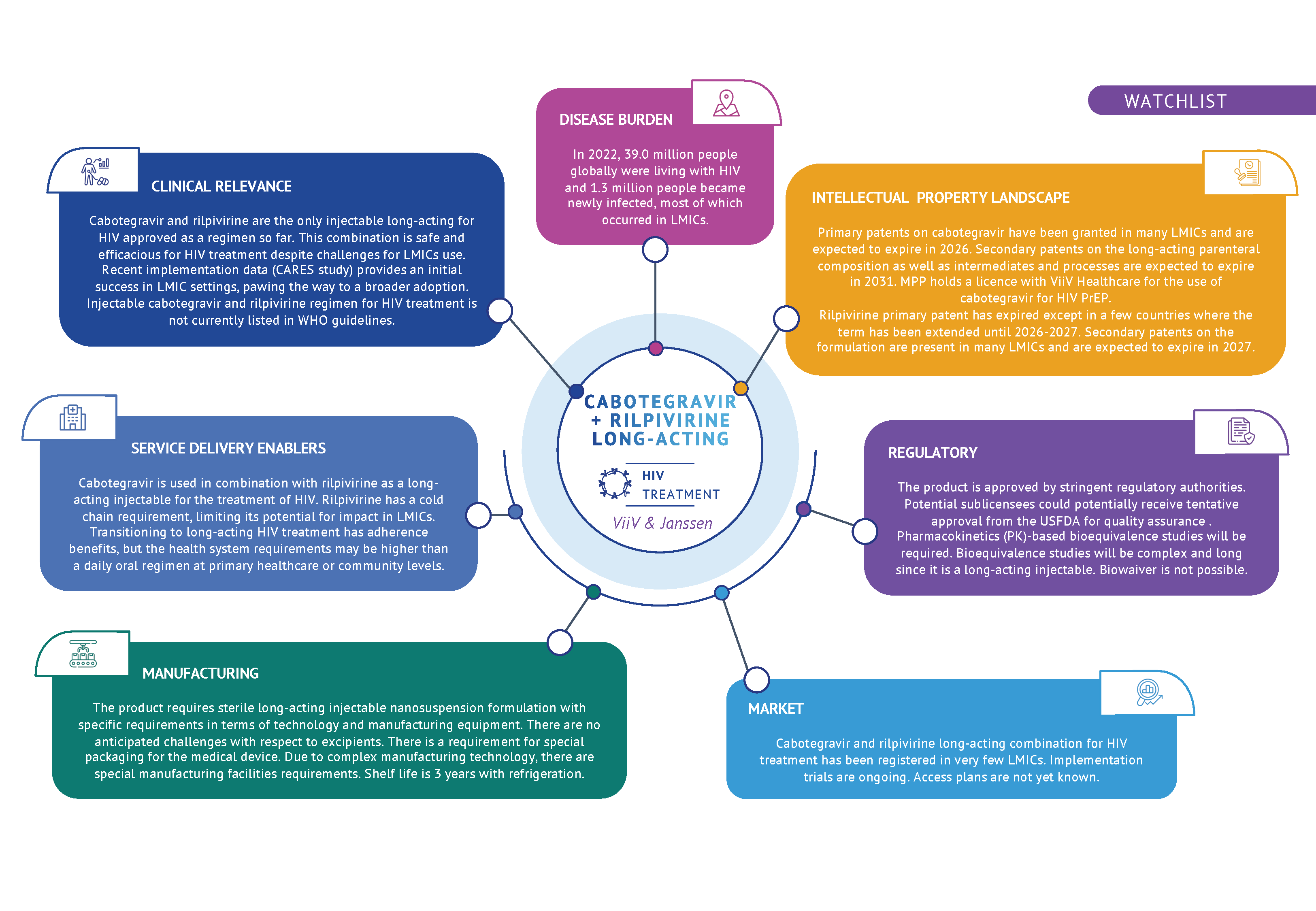
Long-acting cabotegravir for HIV PrEP was licensed to MPP in 2022.
Cabotegravir (CAB) is the only approved integrase strand transfer inhibitor (INSTI) in combination with the non-nucleoside reverse transcriptase inhibitor (NNRTI) rilpivirine (RPV) constituting a fully long-acting treatment for HIV.
This combination regimen is indicated in virologically suppressed adults with no history of treatment failure and with no known or suspected resistance to either cabotegravir or rilpivirine.
This regimen could support treatment adherence and sustain virological suppression in comparison with daily oral treatments. Recent data presented at the 2024 Conference on Retroviruses and Opportunisitic Infections (CROI2024) indicated that people living with HIV with adherence challenges and receiving long-acting injectable cabotegravir and rilpivirine (CAB/RPV) had further reductions in their viral loads than with oral antiretroviral treatment (ART).
Additionally, recent data on the CARES study showed that in sub-Saharan Africa, switching to LA CAB/RPV vs. continuing oral ART yielded high rates of HIV-1 RNA suppression at week 48 (LA CAB/RPV was noninferior to oral ART). This study represents an initial success of such combination in LMIC settings: infrequent HIV-1 RNA monitoring, population with high rates of obesity, prior NNRTI exposure, RPV resistance mutations, and different HIV subtypes compared to other parts of the world. Injectable cabotegravir and rilpivirine regimen for HIV treatment is not currently listed in WHO guidelines for the treatment of HIV.
There might be still be some challenging that could prevent this 2-drugs regimen from being widely implemented in LMICs. Eventual rilpivirine resistance could largely exclude future use of the NNRTI class drugs. Additionally, rilpivirine (not cabotegravir) requires a cold chain and presents some safety issues including hepatic adverse effect.
For people living with hepatitis B, switching from a tenefovir disproxil fumarate (TDF) or tenofovir alafenamide (TAF)-containing regimen (such as oral tenfovir/lamivudine/dolutegravir (TLD)) to CAB/RPV would deprive these individuals from the HBV suppressive action of TDF or TAF. Finally there are some drug-drug interactions with tuberculosis treatment regimens including rifampicin and rifabutin.
Patent & licence data for rilpivirine in LMICs
Patent & licence data for cabotegravir in LMICs
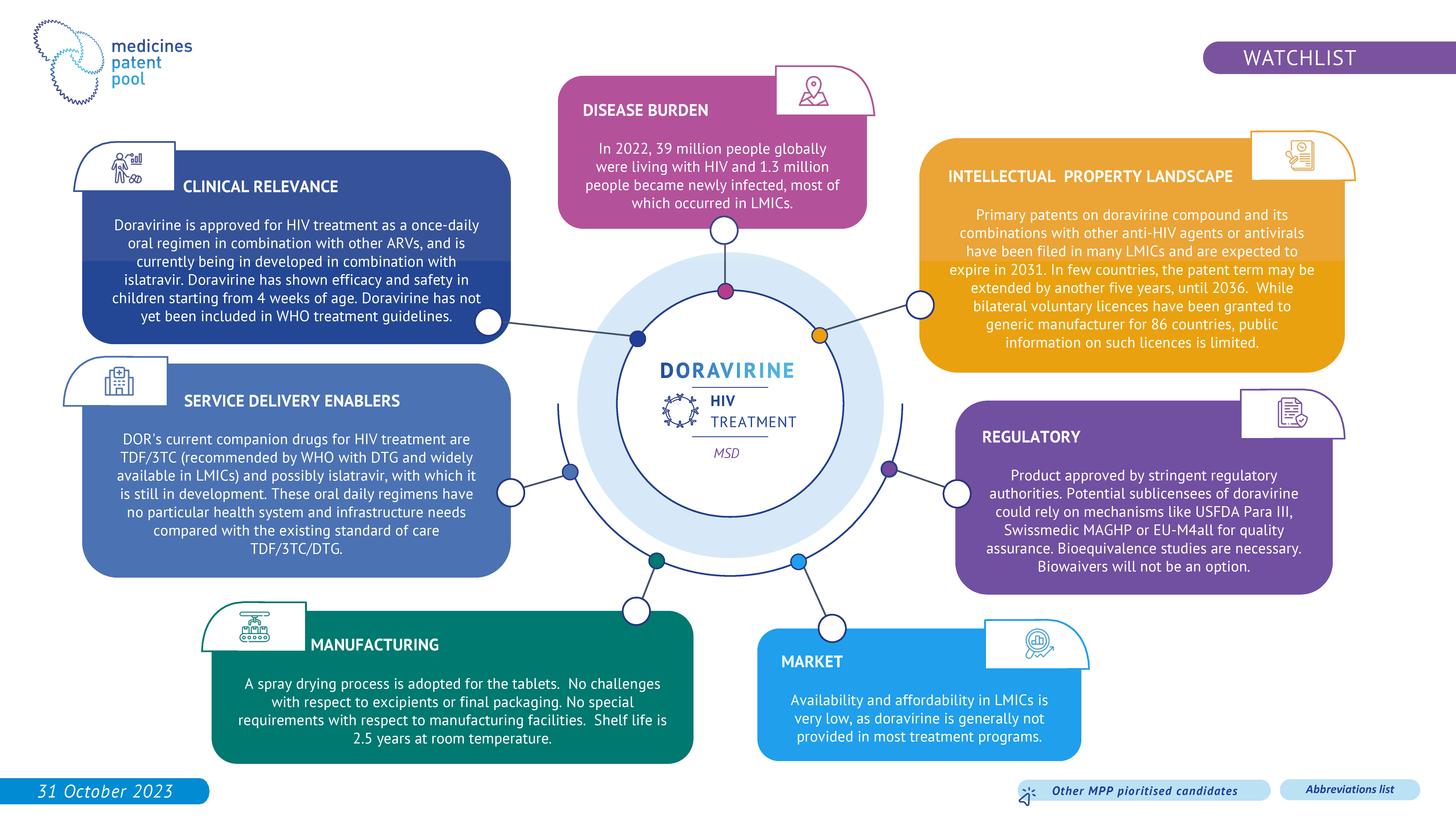
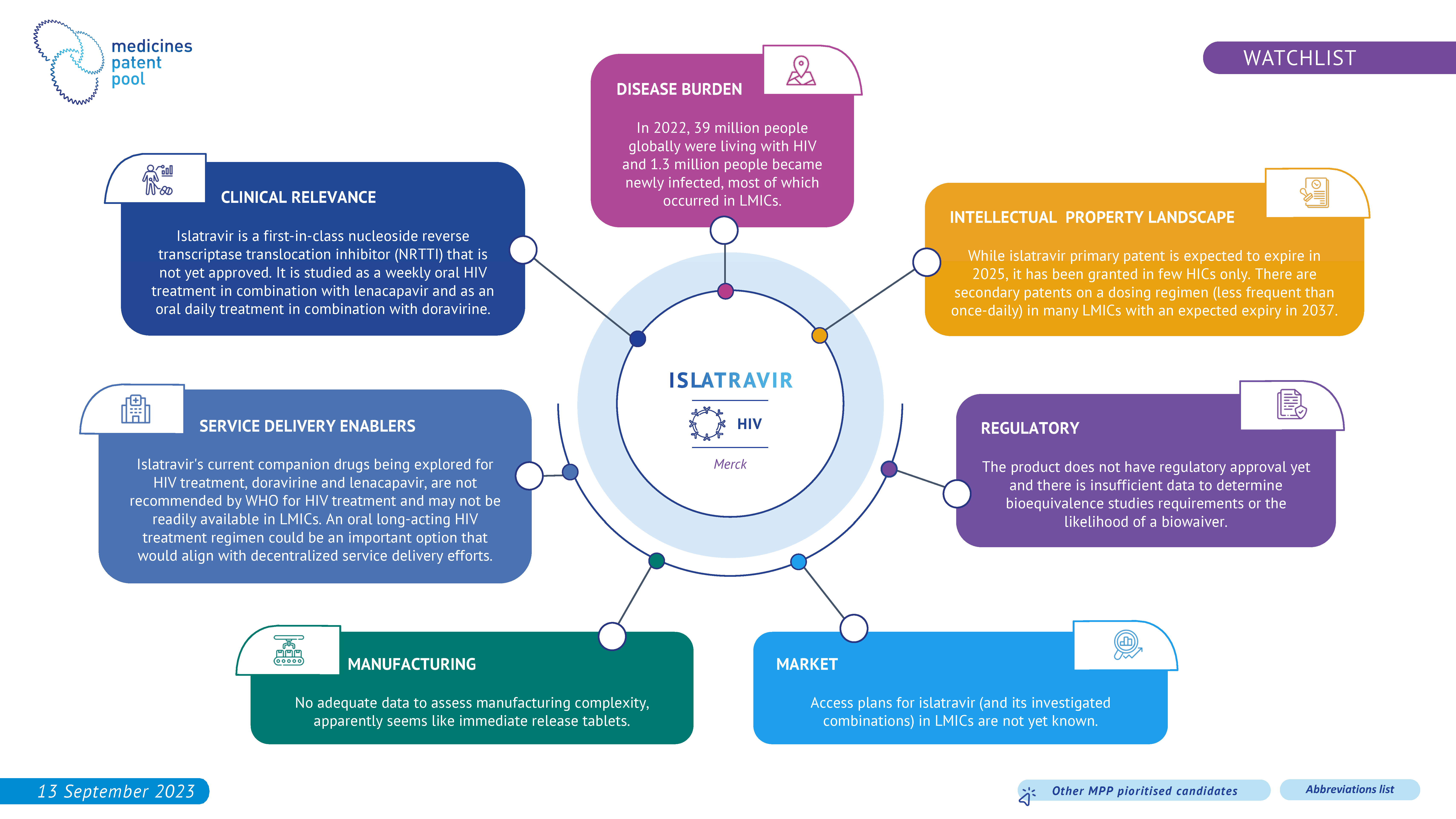
Doravirine is a non-nucleoside reverse transcriptase inhibitor (NNRTI). It is being investigated for HIV treatment in combination with islatravir as once daily oral treatment and it has the potential to be an alternative treatment for PLHIV. Additionally, some of its development program is focused on infants and children. However, the evidence of clinical benefits over the standard of care is unclear.
Islatravir is the first drug of a new class called nucleoside reverse transcriptase translocation inhibitors (NRTTIs). Its new mechanism of action and its long-acting properties have the potential to make it an important product in HIV treatment. After a period of pause in clinical development involving islatravir due to safety concerns, Merck has recently resumed some islatravir’s development programs while monitoring closely its safety. Furthermore, islatravir is one of the most promising drugs studied in combination with lenacapavir for oral weekly treatment and is investigated in co-formulation with other medicines and in several long-acting technology platforms.
These laboratory-made proteins emulate the immune system's ability to counteract harmful pathogens, including HIV. Although still in the early stages of development, broadly-neutralising antibodies demonstrate significant potential for use in post-natal prophylaxis (PNP) against HIV, due to their favourable safety profile and the convenience they may offer through a single administration via intramuscular injection.
354 million people globally live with a hepatitis B or C infection1. Hepatitis causes liver damage and cancer. It kills over a million people annually. Of the 5 types of hepatitis infections, hepatitis B and C cause most of the disease and deaths. Hepatitis C can be cured; however, only 21% of people living with hepatitis C infection are diagnosed and only 13% have received curative treatment. MPP works with a wide range of partners including originator and generic companies, governments, WHO, civil society and communities2, procurement agencies, and others to expand and accelerate the development and distribution of these new treatments that can eliminate the virus through a short course of oral therapy in LMICs with a high HCV burden.
Hepatitis C
Hepatitis C is a liver disease caused by the hepatitis C virus (HCV). It is estimated that among the 58 million individuals living with hepatitis C virus, 62% of them reside in low- and middle-income countries3. New direct-acting antivirals (DAA) that are effective across all major HCV genotypes can cure more than 95% of the people treated, generally in 3 months or less. Yet approximately 84% of the people infected with HCV are not receiving treatment4. The Medicines Patent Pool works with generic partners to speed the development and distribution of these new treatments that can eliminate the virus through a short course of oral therapy in regions with a high HCV burden. MPP signed licence agreements for three hepatitis C treatments: daclatasvir (DAC) in 2015, ravidasvir (RAV) in 2017 and glecaprevir/pibrentasvir (G/P) in 2018. Moreover, a long-acting injectable formulation of G/P is being developed by University of Liverpool with Unitaid funding, and has been licensed to MPP.
Hepatitis B
Chronic hepatitis B is a disease affecting 296 million globally4. Around 60% of people living with hepatitis B live in LMICs. MPP’s licences from Gilead Sciences, covering tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF), benefit people living with HIV as well as people living with chronic hepatitis B.
1 World Health Organization - Elimination of hepatitis by 2030
2 In the framework of MPP’s work with communities, MPP relies on its Community Advisory Panel, a group of experts with lived experience that support MPP in the implantation of our activities.
3 World Health Organization - Fact sheet – Viral hepatitis B and C policies in countries and burden of disease in WHO regions, 2023
4 World Health Organization - Global report on HIV, viral hepatitis and sexually transmitted infections, 2021
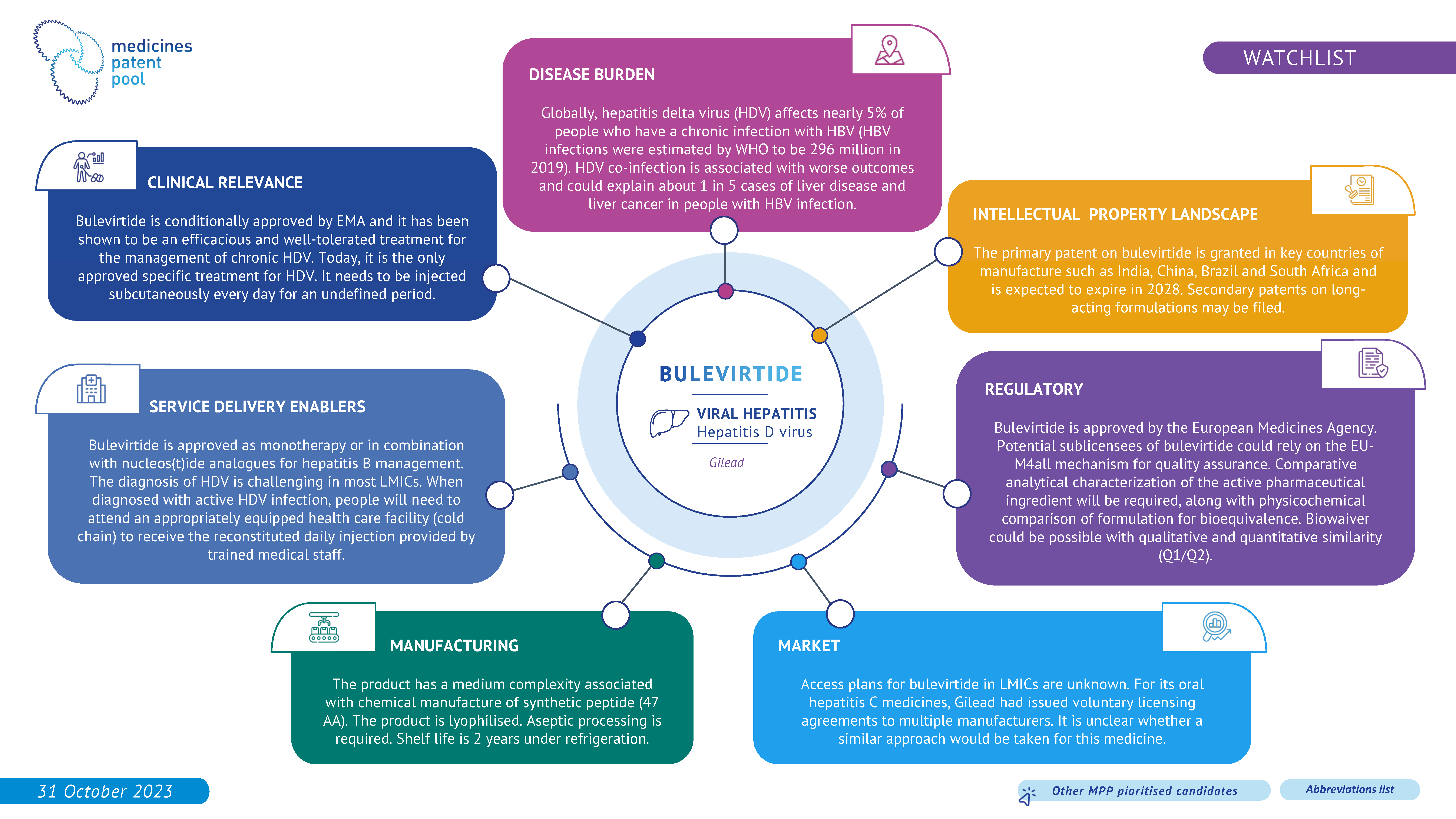
Bulevirtide was recently conditionally approved by EMA and is today the only specific treatment for hepatitis delta. The treatment's duration and the necessity of a daily subcutaneous injection may present some challenges in LMICs. Additionally, its safety and efficacy are still under evaluation and therefore bulevirtide has been included in the watchlist.
An estimated global total of 10.6 million people fell ill with tuberculosis (TB) in 2021, most in low- and middle-income countries. TB is the leading cause of death for people with HIV and a major contributor to antimicrobial resistance. In 2021, 1.6 million people died from TB, including 187 000 people with HIV. Globally, there were an estimated 450 000 incident cases of multidrug-resistant or rifampicin-resistant tuberculosis (MDR/RR-TB) in 2021.
The World Health Organization’s post-2015 Global TB Strategy1 sets ambitious targets ed at reducing TB deaths by 95% between 2015 and 2035, and to end TB. To meet these targets, better therapies to treat TB are urgently needed, particularly for MDR-TB. Since 2020, PAN-TB, Project to Accelerate New Treatments for Tuberculosis (both drug sensitive and multidrug-resistant TB) aims at developing new drug regimens to transform the care of patients with tuberculosis.
We work to improve access to new treatments for MDR-TB and drug-susceptible tuberculosis. We also facilitate the development of new regimens by licensing TB drugs that are still under development. In early 2017, MPP signed its first agreement with the Johns Hopkins University. This agreement was to facilitate the clinical development of sutezolid, a promising investigational treatment for tuberculosis. It was followed by a second agreement with Pfizer in October 2019 to access Pfizer’s preclinical, phase I and phase IIa clinical study data and results on sutezolid. The agreement’s aim was to further study, develop and make available this potential important component of new TB regimens.
1 World Health Organization - Global Tuberculosis Report 2022 (last accessed on 25 Sept. 2023).
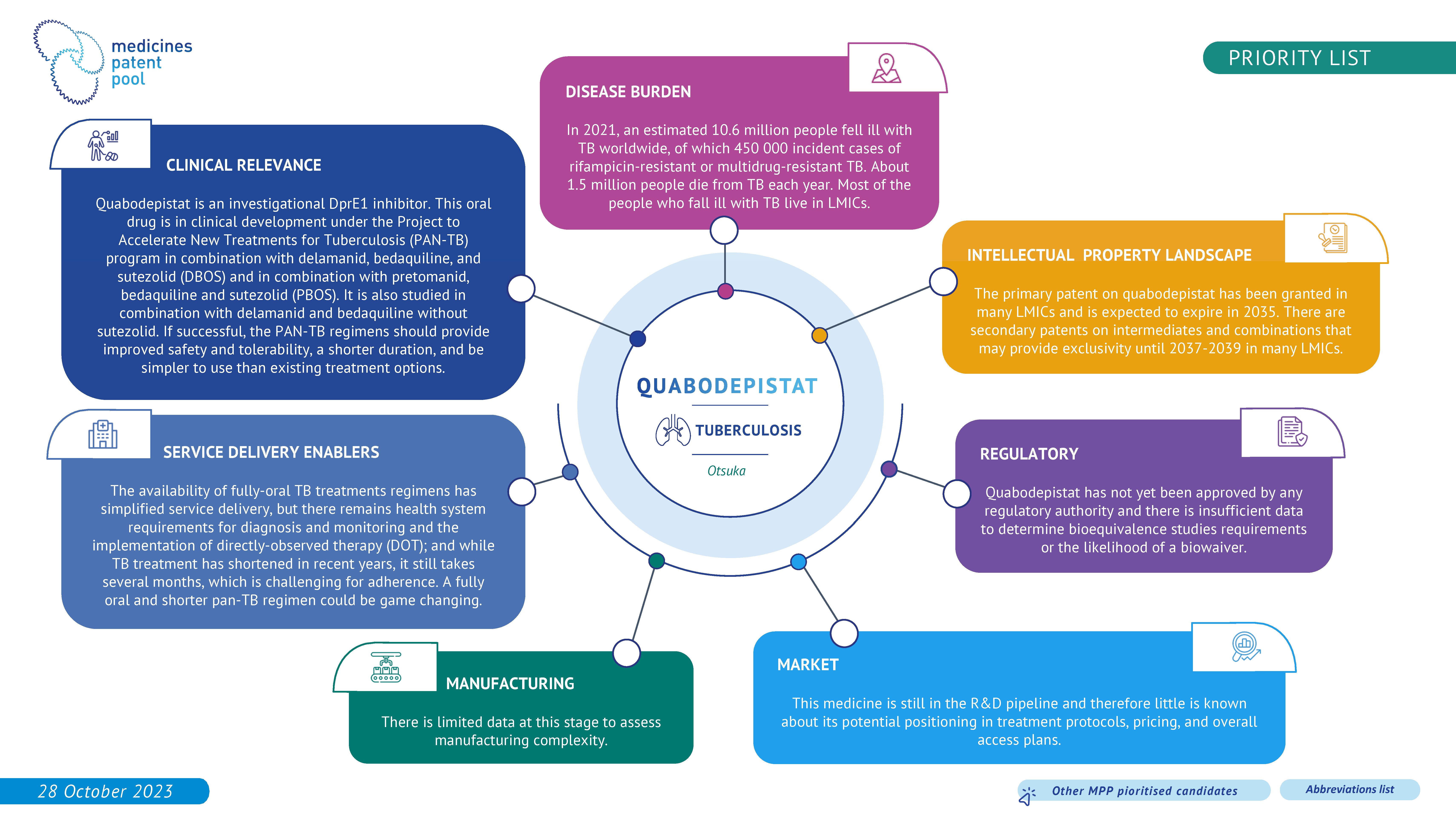
Quabodepistat (OPC-167832) is a molecule with a new mechanism of action that has potent antituberculosis activity and a favourable safety profile. It is now studied as part of a new, promising, TB regimen under the Project to Accelerate New Treatments for Tuberculosis (PAN-TB) program, in combination with delamanid, bedaquiline, and sutezolid (DBOS) or in combination with pretomanid, bedaquiline and sutezolid (PBOS).
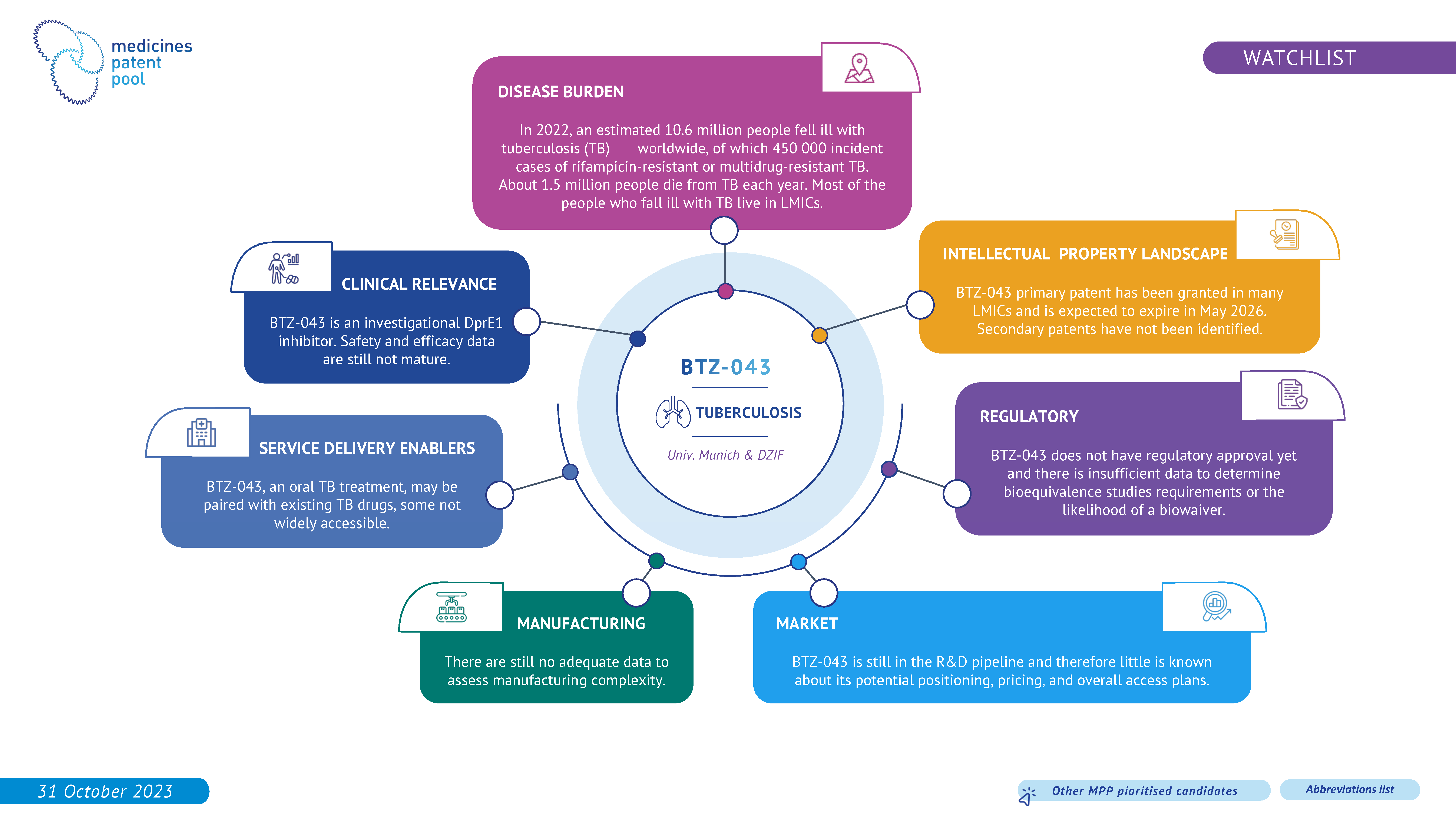
BTZ-043 is an investigational agent, active against all tested Mycobacterium tuberculosis strains, including clinical isolates from MDR and XDR patients. The clinical data on BTZ-043 are still immature and therefore the drug candidate remains on the watchlist.
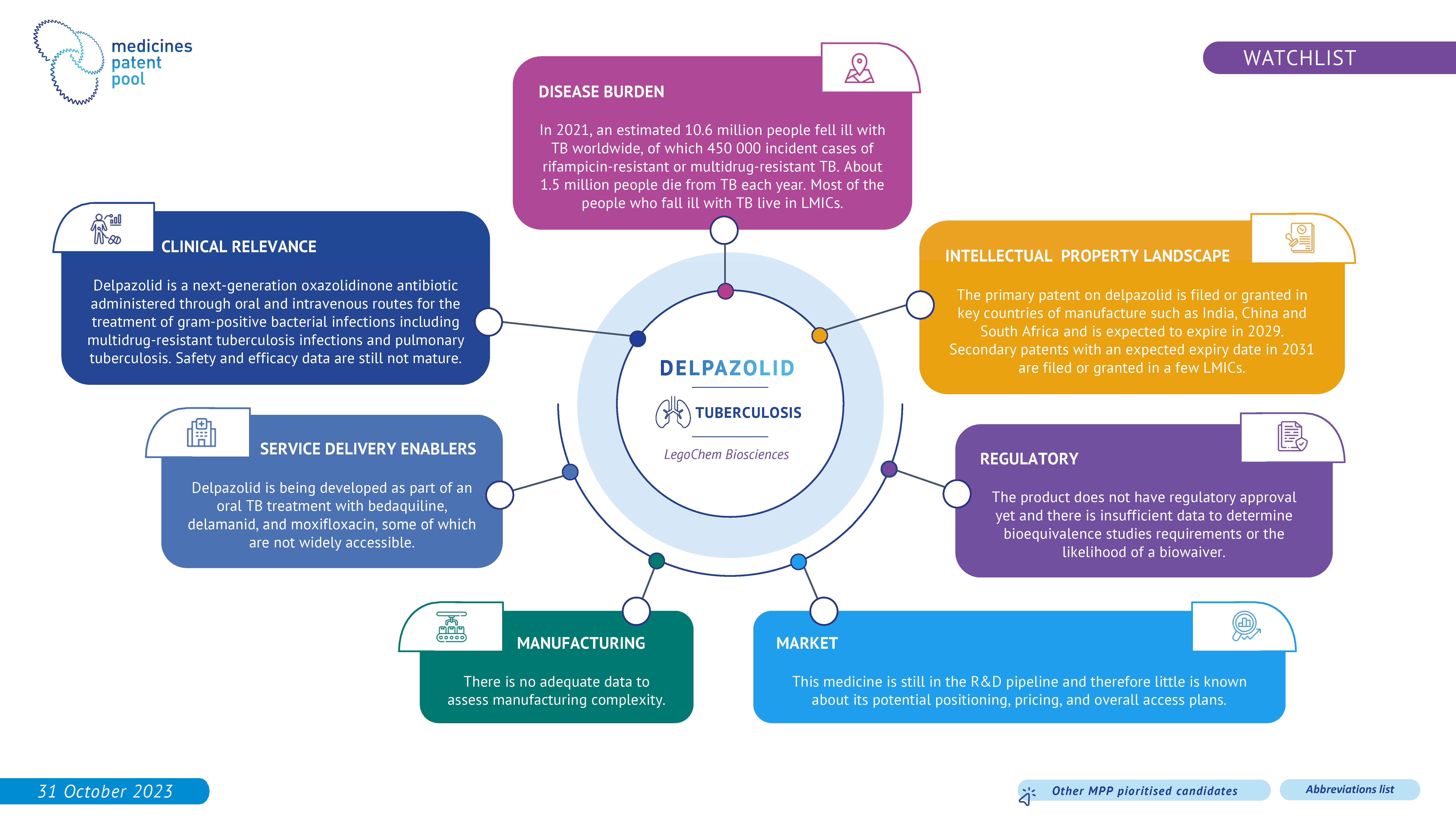
Delpazolid is an investigational antitubercular agent. It is being studied in combination with delamanid and bedaquiline. As the clinical data are still immature, the drug candidate remains in the watchlist.
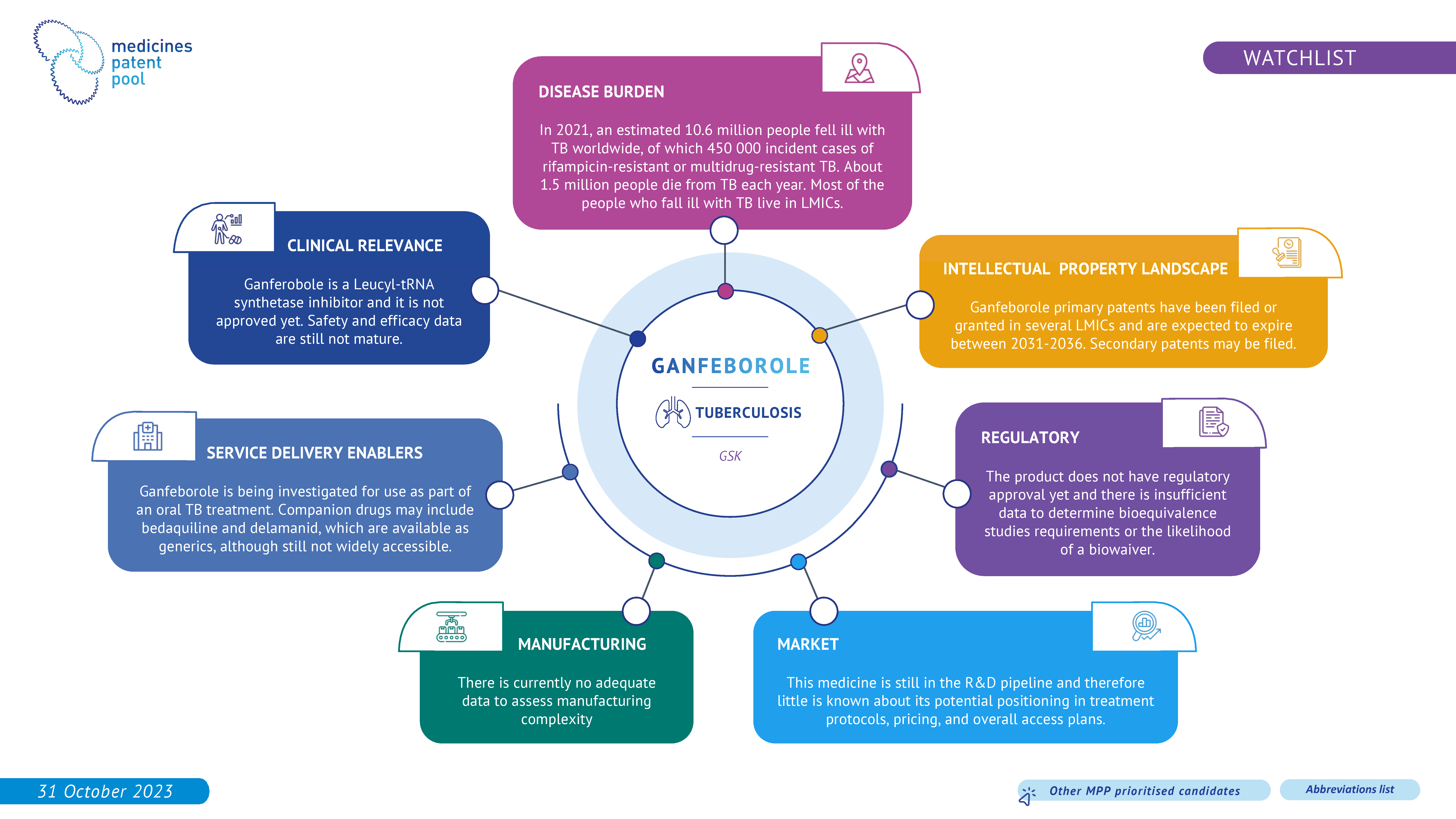
Ganfeborole (GSK3036656) is an investigational agent, demonstrating early bactericidal activity with a low, once-daily oral dose after 14 days of treatment in participants with drug-susceptible pulmonary tuberculosis. The clinical data on GSK3036656 are still immature and therefore the drug candidate remains in the watchlist.
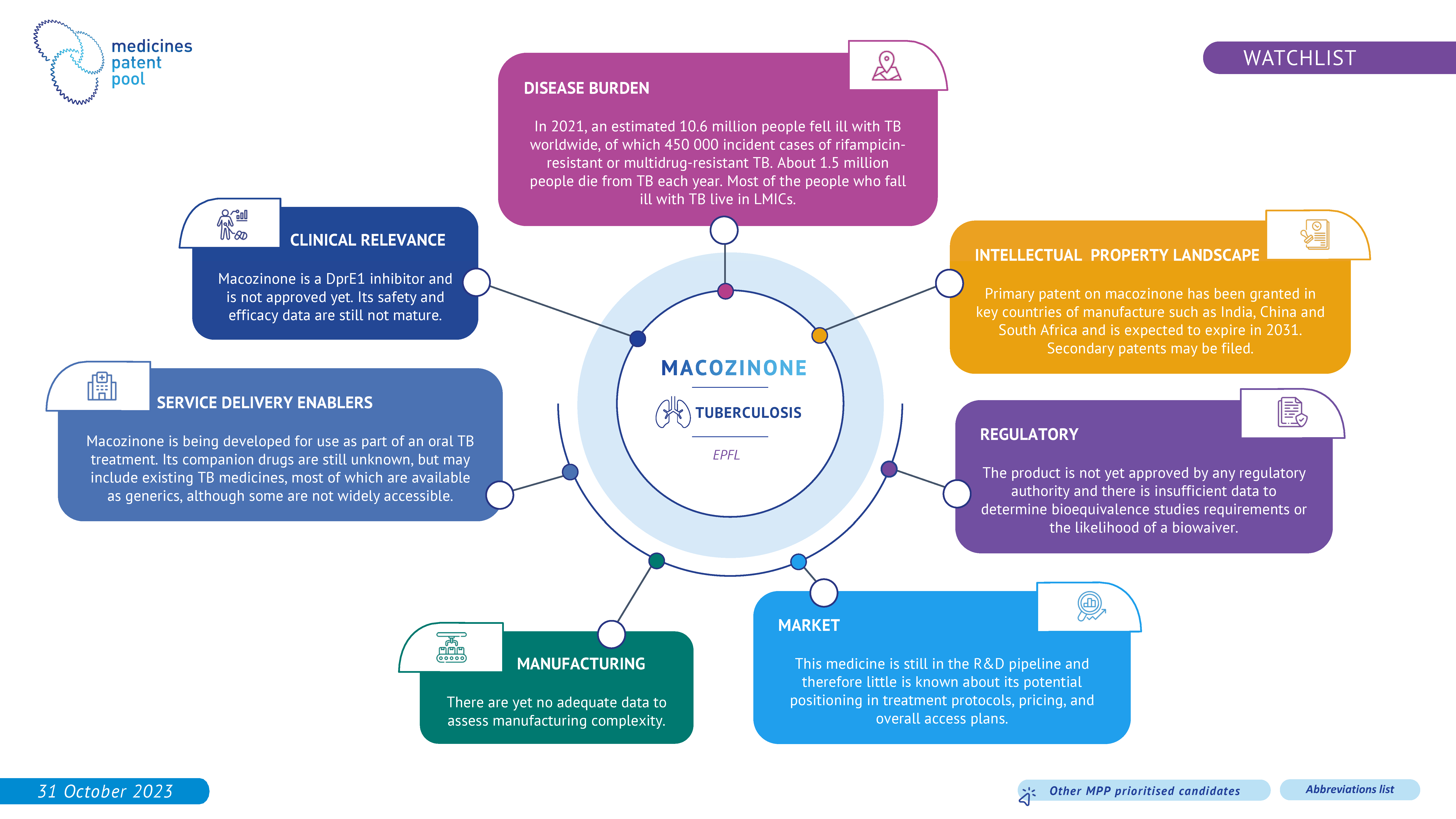
Macozinone (PBTZ-169) is a tuberculosis drug candidate that has demonstrated high potency against drug-susceptible and drug resistant Mycobacterium tuberculosis in pre-clinical studies. Macozinone has additive effects with many tuberculosis therapeutic agents, both marketed and in development, and has synergic effects with bedaquiline and clofazimine in preclinical models. Clinical data on macozinone is still immature.
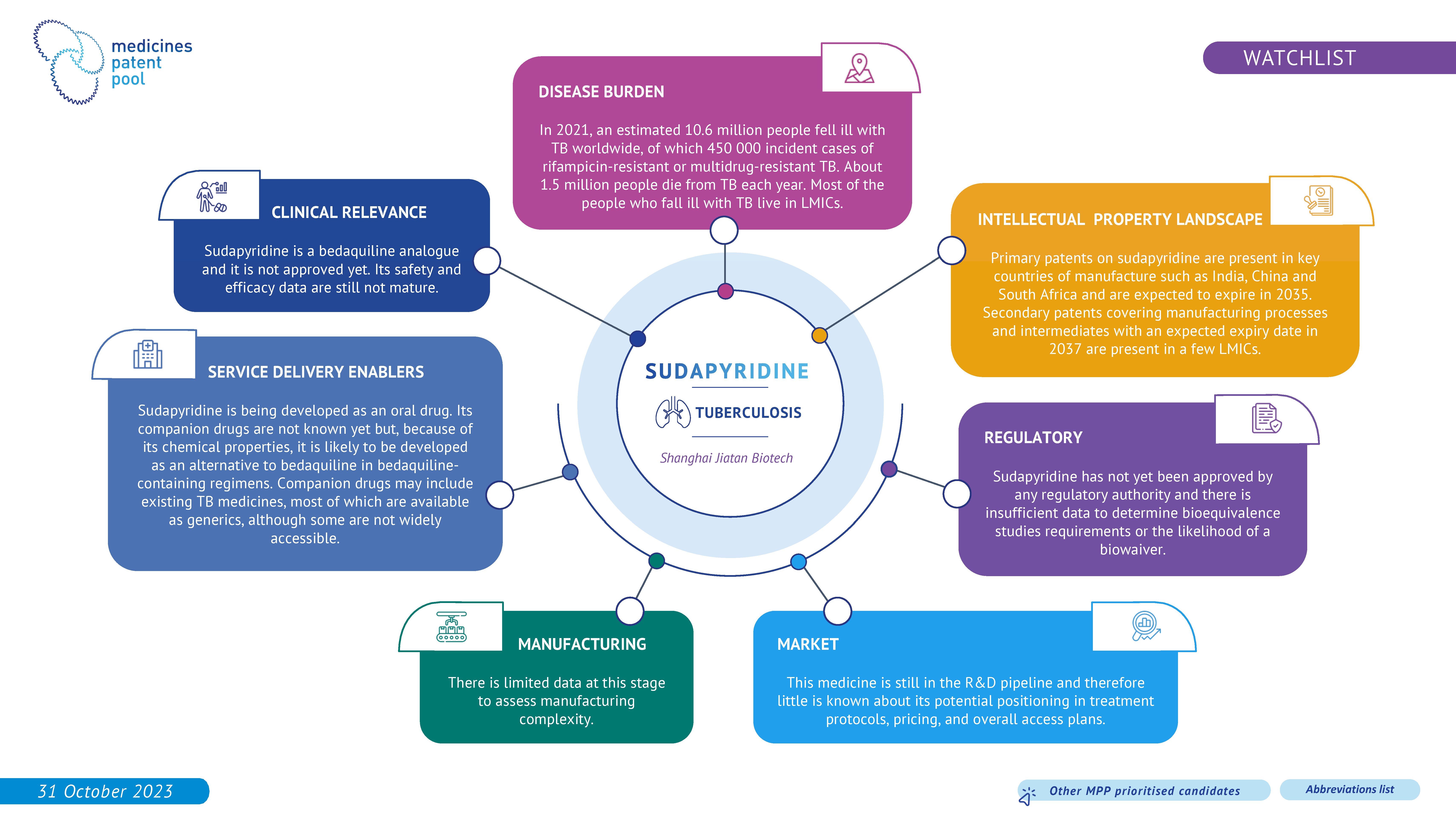
Sudapyridine (WX-081) is a bedaquiline analogue, displaying antimycobacterial activity and low toxicity. Clinical data on sudapyridine is still unmature.
Pandemic and epidemic threats
Epidemics are an unexpected, often sudden, increase of a specific illness within a community or region. Pandemics are when an epidemic occurs worldwide, crossing international borders and affecting a large number of people. A number of communicable diseases can be significant health threats at the local, regional and global level and lead to epidemics or pandemics. Epidemics and pandemics can be prevented and mitigated through a range of measures, such as good hygiene, social distancing, medicines and vaccination.
Pandemics pose a significant threat to global health security. By actively engaging in pandemic preparedness, MPP contributes to the resilience of healthcare systems and helps mitigate the impact of future health crises. MPP works with innovators and pharmaceutical companies to expedite the development and manufacturing of medical countermeasures.
By facilitating intellectual property licensing and technology transfer, MPP can support the production of these life-saving tools in LMICs and contribute to ensuring that pandemic-related products are accessible to governments and healthcare systems, especially in low- and middle-income countries, in an equitable manner.
Influenza
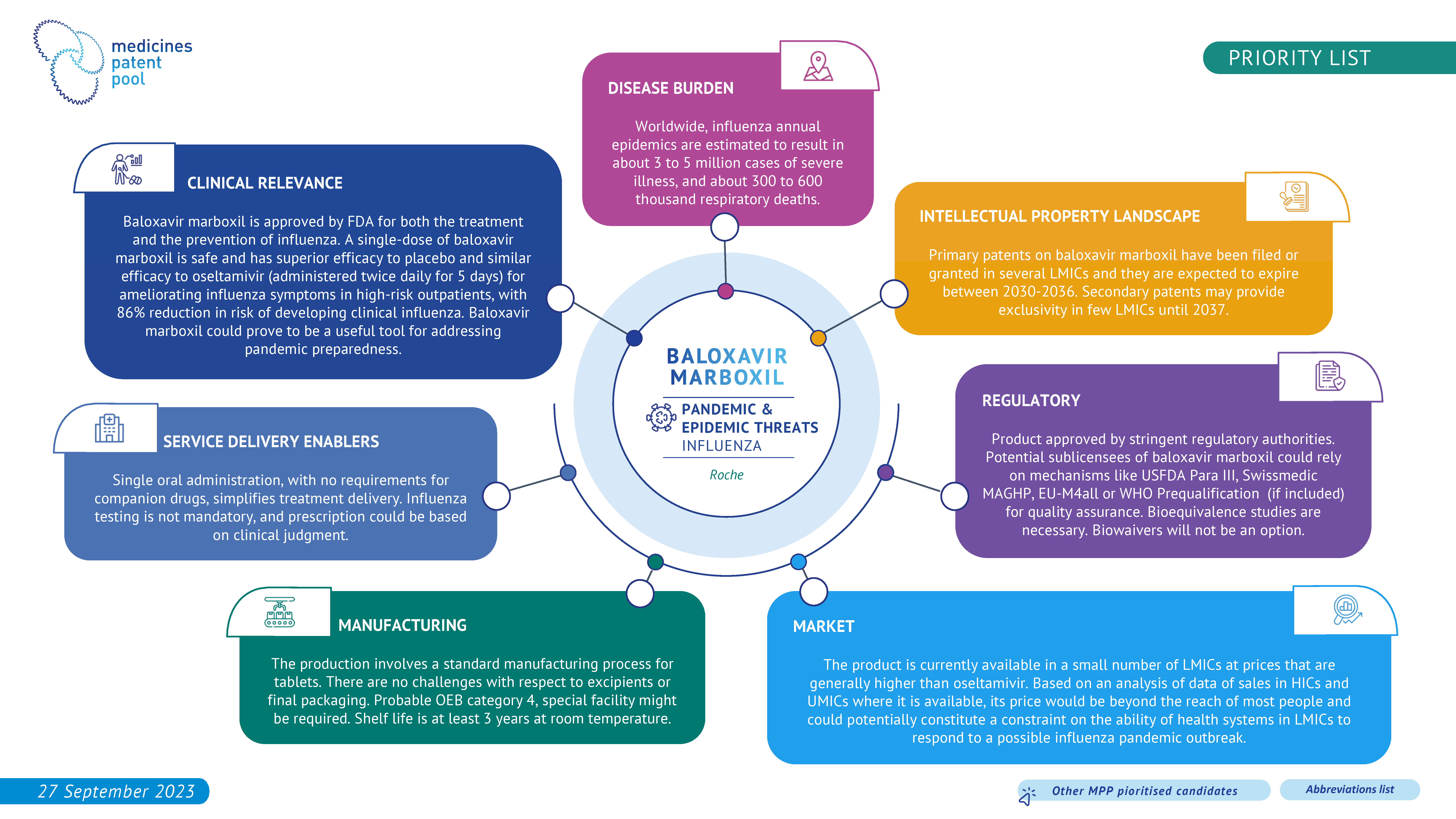
Seasonal influenza is an acute respiratory infection caused by influenza viruses which circulate in all parts of the world. It represents a year-round disease burden. It causes illnesses that range in severity and sometimes lead to hospitalization and death.
Worldwide, influenza epidemics are estimated to result in about 3 to 5 million cases of severe illness, and about 290 000 to 650 000 respiratory deaths annually.
Given its potential impact on eventual influenza pandemics, MPP has added baloxavir marboxil as a priority product for in-licensing.
INFLUENZA
Baloxavir marboxil, an oral one-dose, one-time treatment for influenza, could serve, in addition to its use in seasonal influenza, as a valuable tool in pandemic preparedness in the event of a novel and highly virulent influenza strain. As a drug in a new class of antiviral treatment for influenza, it could provide an additional layer of defense and an option against viruses resistant to existing antivirals.
Antimicrobial resistance (AMR) threatens the effective prevention and treatment of an ever-increasing range of infections caused by bacteria, parasites, viruses and fungi.
AMR occurs when bacteria, viruses, fungi and parasites evolve over time and no longer respond to medicines making infections harder to treat and increasing the risk of disease spread, severe illness and death. As a result, the medicines become ineffective and infections persist in the body, increasing the risk of spread to others.
Antimicrobials - including antibiotics, antivirals, antifungals and antiparasitics - are medicines used to prevent and treat infections in humans, animals and plants. Microorganisms that develop antimicrobial resistance are sometimes referred to as “superbugs”.
MPP’s commitment to identify promising new medicines for infectious diseases caused by pathogens which have developed resistance against available therapies, is balanced with stewardship efforts to avoid development of resistance to new antimicrobials. This is an area in which MPP has already undertaken work in the context of its TB activities, and it will continue to support ongoing efforts to combat antimicrobial resistance.
Gepotidacin is an investigational first-in-class oral antibiotic for treatment of uncomplicated urinary tract infections and of Uncomplicated Urogenital Gonorrhea (UUG). Gepotidacin is active against most strains of target uropathogens, such as E.coli and Staphylococcus saprophyticus, including isolates resistant to current antibiotics. Furthermore, due to gepotidacin’s mechanism of action, blocking bacterial DNA replication by inhibiting two vital enzymes, mutations in both enzymes are needed to significantly affect susceptibility to gepotidacin. This gives hope that this drug will stay effective as resistance would be harder to develop.
Cancer is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020, or nearly one in six deaths1. Among the prevalent cancer types, breast, lung, colon and rectum, prostate, stomach, liver, cervix uteri and skin cancers stood out as the most frequently encountered. Many cancers can be cured if detected early and treated effectively. Many health systems in low- and middle-income countries remain ill-equipped to cope with this growing health crisis. Consequently, a significant portion of cancer patients worldwide continues to face significant barriers to accessing timely and high-quality diagnosis and treatment.
Lung cancer
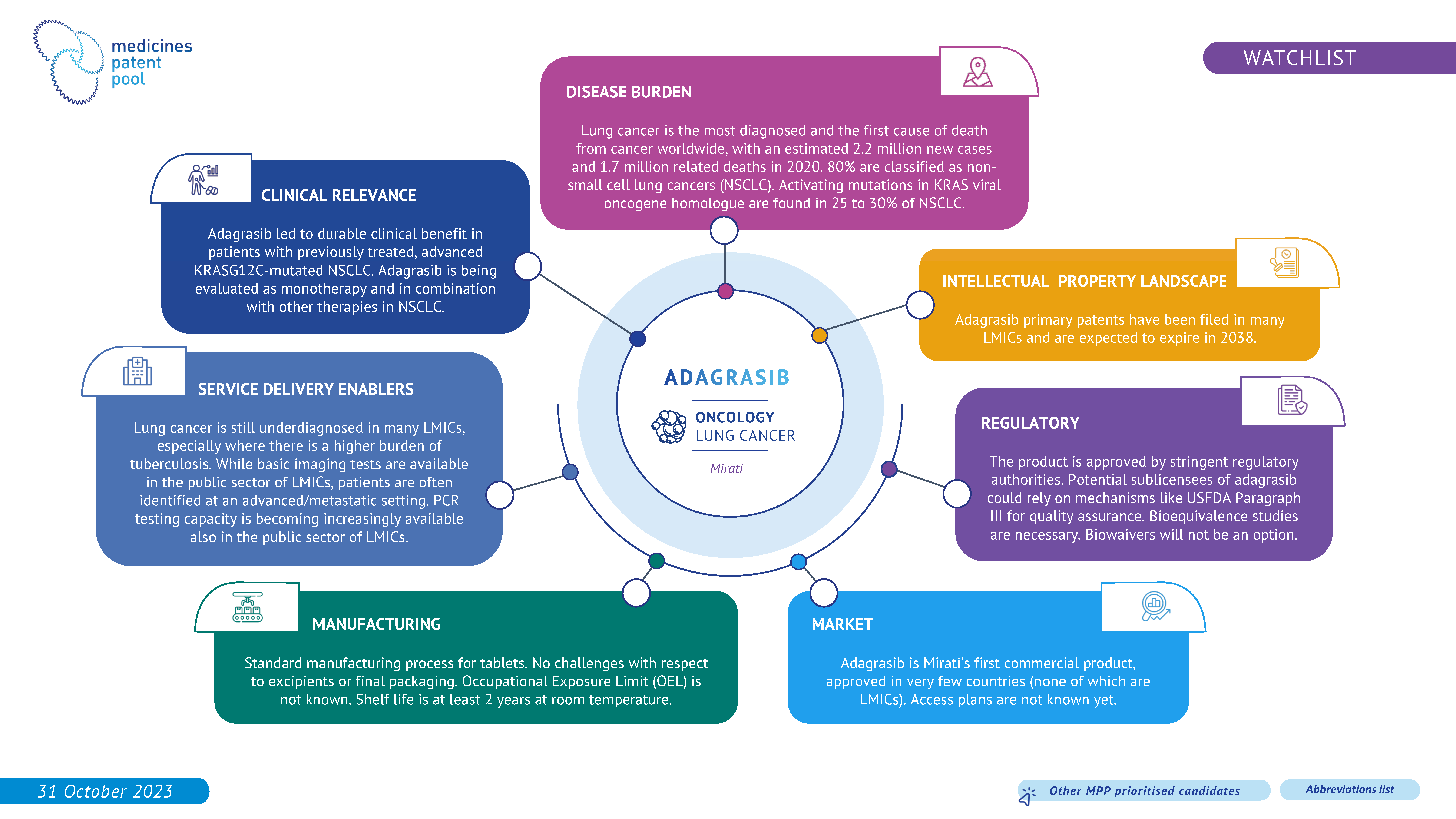
Lung cancer arises from the rapid and unregulated proliferation of abnormal cells in the lungs, posing a significant threat to health and carrying a high risk of fatality. The two most common forms of lung cancer are: non-small cell lung carcinoma (NSCLC), which is prevalent and tends to develop at a gradual pace, and small cell lung carcinoma (SCLC), which is rarer but usually exhibits a rapid growth rate. In low- and middle-income countries (LMICs), there were 1.2 million incident cases of non-small cell lung cancer (NSCLC) in 20202. Unfortunately, around 70% of NSCLC cases are diagnosed in advanced stages, such as locally advanced or metastatic.
Breast cancer
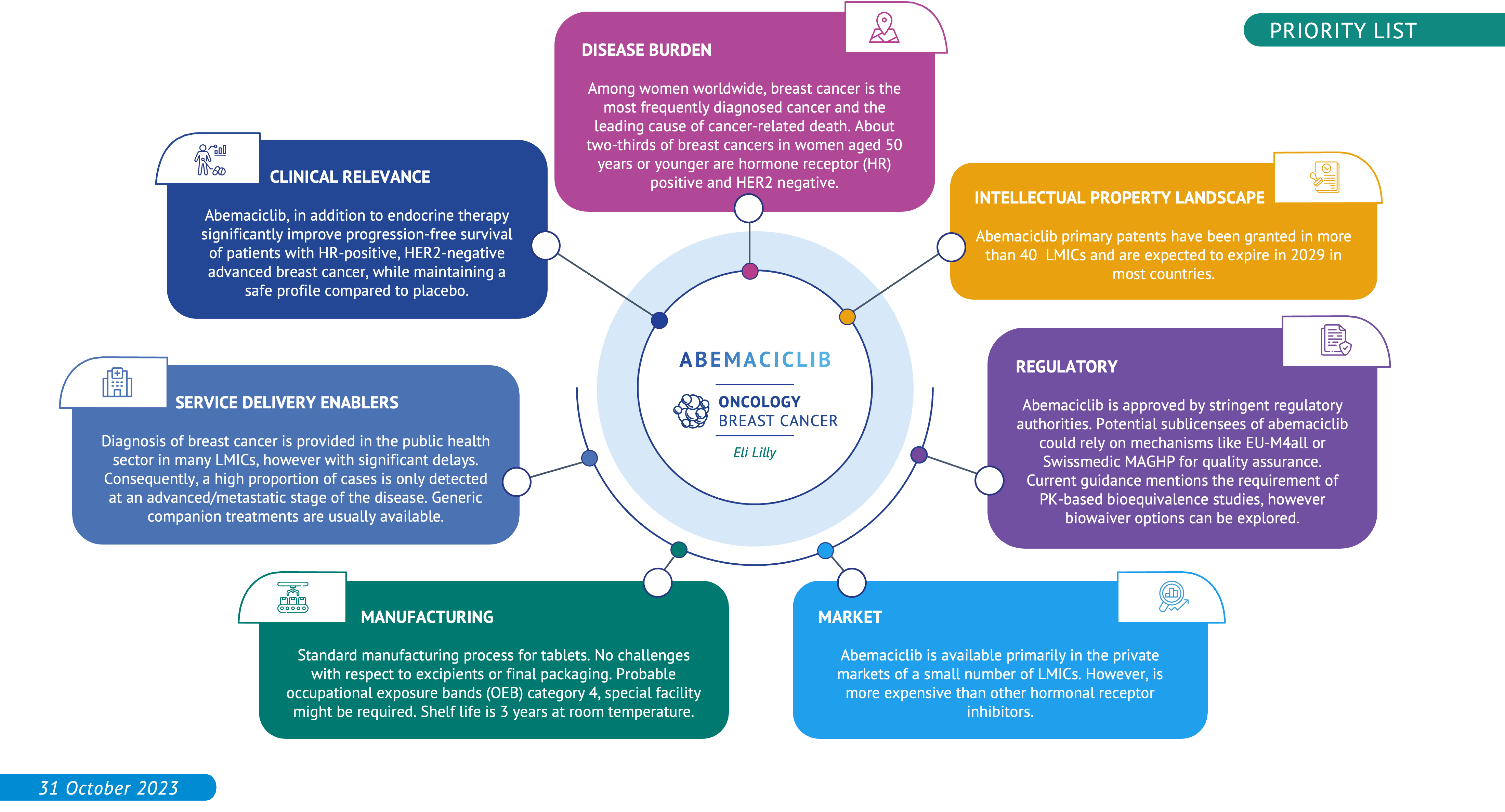
Breast cancer is the most commonly occurring cancer in women and the most common cancer overall. There were more than 2.26 million new cases of breast cancer in women in 20203. There are four main female breast cancer subtypes, including the following in order of prevalence: HR+/HER2-, HR-/HER2-, HR+/HER2+, HR-/HER2+. HR stands for hormone receptor. HR+ means that tumour cells have receptors for the hormones estrogen or progesterone, which can promote the growth of HR+ tumours. HER2 stands for human epidermal growth factor receptor 2. HER2+ means that tumour cells make high levels of a protein called HER2/neu, which has been shown to be associated with certain aggressive types of breast cancer4.
Chronic lymphocytic leukaemia
Chronic Lymphocytic Leukaemia (CLL) is the most common form of leukaemia, accounting for 25% to 30% of all leukaemia cases in Western countries5. In 2019, CLL resulted in 44,612 deaths worldwide. Regarding incidence rates in 2019, CLL affected 1.34 individuals per 100,000 in the global population, with rates of 1.13 in high-income countries, 0.45 in middle-income countries, and 0.28 in low-income countries6. CLL predominantly occurs in older individuals, peaking in the elderly population, with a median age at diagnosis of 71 years in Europe7. Furthermore, the incidence of CLL is approximately twice as high in males compared to females8.
Prostate cancer
Prostate cancer made up 7.3% of all new cancer cases in 2020, accounting for 14.1% of all new male cancer diagnoses9. Notably, cancer incidence rates have been on the rise in various Sub-Saharan African populations10. Men of African descent face nearly double the risk of being diagnosed with prostate cancer before the age of 45 compared to Caucasian men11. Additionally, research indicates that men with greater overall and central body fat have a heightened risk of prostate cancer-related mortality12.
1 World Health Organization- Cancer Fact Sheet (last accessed 25 Sept. 2023).
2 International Agency for Research on Cancer - Cancer Today (last accessed 25 Sept. 2023).
3 International Agency for Research on Cancer - Cancer Today (last accessed 25 Sept. 2023).
4 NIH - National Cancer Institute (last accessed 25 Sept. 2023).
5 The global burden and attributable risk factors of chronic lymphocytic leukemia in 204 countries and territories from 1990 to 2019: analysis based on the global burden of disease study 2019. Biomed Eng Online. 2022.
6 Institute for Health Metrics and Evaluation - Global Burden of Disease Study 2019.
7 Management of chronic lymphocytic leukemia (CLL) in the elderly: a position paper from an international Society of Geriatric Oncology (SIOG) Task Force. Annals of Oncology. 2017.
8 Sex differences in incidence and outcome of chronic lymphocytic leukemia patients. Leukemia & Lymphoma. 2006.
9 International Agency for Research on Cancer - Cancer Today (last accessed 25 Sept. 2023).
10 Rising Prostate Cancer Incidence in Sub-Saharan Africa: A Trend Analysis of Data from the African Cancer Registry Network. Cancer Epidemiol Biomarkers Prev. 2021.
11 Pathways to diagnosis for Black men and White men found to have prostate cancer: the PROCESS cohort study. Br J Cancer. 2008.
12 Adiposity and risk of prostate cancer death: a prospective analysis in UK Biobank and meta-analysis of published studies. BMC Med. 2022.
MULTIPLE CANCER INDICATIONS
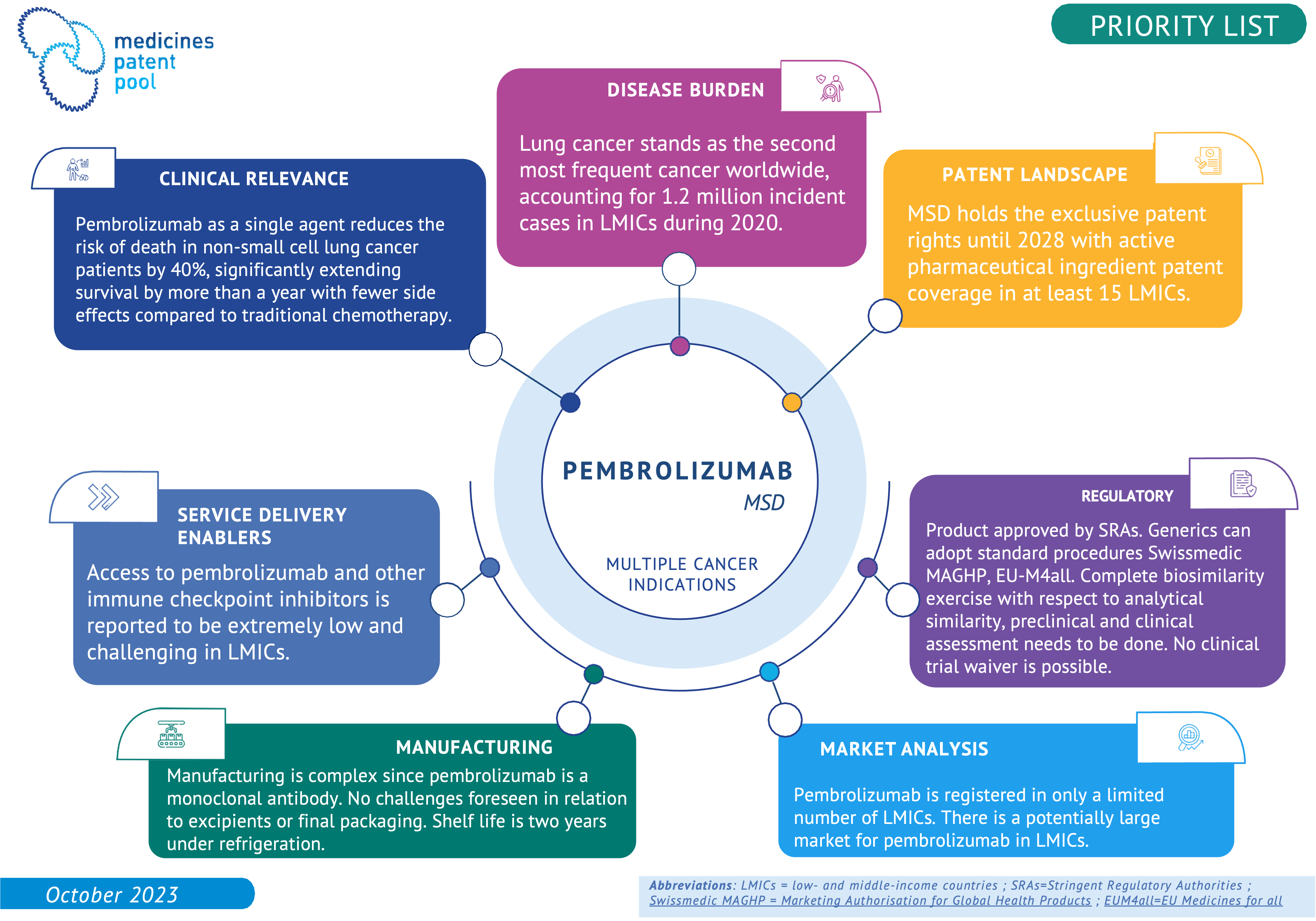
Immune check-point inhibitors (ICIs) are monoclonal antibodies, used for immunotherapy in the oncology field. ICIs block proteins that stop the immune system from attacking the cancer cells. Most known class representatives are PD-1 inhibitors and PD-L1 inhibitors. ICIs have been approved to treat a variety of cancers and many ICIs are under development. The WHO Essential Medicines List (EML) committee recognised their importance as a therapeutic class. Some of them are listed on the EML for the treatment of skin melanoma. In several cases, the WHO EML Committee recommended to continue working on strategies to improve the affordability of these medicines and also suggested some for consideration for licensing by MPP. Moreover, MPP technology transfer support could support a successful implementation by reducing development time and costs of biosimilar versions for use in LMICs. MPP has prioritized ICIs as a class, and specific molecules, such as pembrolizumab, are listed here as examples of this effort.
The intravenous formulation of paclitaxel was added to the EML in 2011 and used in the treatment protocols for many cancers. Pending confirmation of its safety and efficacy, this new mode of oral administration, currently under development, could be particularly promising for LMIC settings, and therefore have been included in the watchlist.
LUNG CANCER
Immune check-point inhibitors (ICIs) are monoclonal antibodies, used for immunotherapy in the oncology field. ICIs block proteins that stop the immune system from attacking the cancer cells. Most known class representatives are PD-1 inhibitors and PD-L1 inhibitors. ICIs have been approved to treat a variety of cancers and many ICIs are under development. The WHO Essential Medicines List (EML) committee recognised their importance as a therapeutic class. Some of them are listed on the EML for the treatment of skin melanoma. In several cases, the WHO EML Committee recommended to continue working on strategies to improve the affordability of these medicines and also suggested some for consideration for licensing by MPP. Moreover, MPP technology transfer support could support a successful implementation by reducing development time and costs of biosimilar versions for use in LMICs. MPP has prioritized ICIs as a class, and specific molecules, such as pembrolizumab, are listed here as examples of this effort.
One of the immune checkpoint inhibitors prioritised by MPP is pembrolizumab. Pembrolizumab as a single agent reduces the risk of death in non-small cell lung cancer patients by 40%, significantly extending survival by more than a year with fewer sides effects compared to traditional chemotherapy. Pembrolizumab is also indicated against several other cancers.
Aumolertinib is a 3rd generation Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor (EGFR TKI), that has demonstrated significant benefits compared to standard first-line treatments of 1st and 2nd generations in the WHO Essential Medicines List for the treatment of non-small cell lung cancer. Aumolertinib is under review for approval by the European Medicines Agency (EMA).
Osimertinib is a 3rd generation Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor (EGFR TKI) approved by the Food and Drug Association and the European Medicines Agency . It has demonstrated significant benefits compared to standard first-line treatments of 1st and 2nd generations in the WHO Essential Medicines List (EML) for the treatment of non-small cell lung cancer. The EML committee called on MPP to explore licensing opportunities for osimertinib.
Adagrasib is an oral Kirsten Rat Sarcoma Virus (KRAS) inhibitor that received approval by the Food and Drug Administration (FDA) for the treatment of non-small cell lung cancer (NSCLC) in 2022 and showcases durable clinical benefits in patients.
Lazertinib is an investigational 3rd generation Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor (EGFR TKI), presenting superiority over the 1st generation of EGFR TKI. Lazertinib has a strong potential as alternative option for lung cancer treatment.
The intravenous formulation of paclitaxel was added to the EML in 2011 and used in the treatment protocols for many cancers. Pending confirmation of its safety and efficacy, this new mode of oral administration, currently under development, could be particularly promising for LMIC settings, and therefore have been included in the watchlist.
Sotorasib is an oral Kirsten Rat Sarcoma Virus (KRAS) inhibitor, that received accelerated approval by the Food and Drug Administration (FDA) for the treatment of non-small cell lung cancer (NSCLC) in 2021. A recent study showed that sotorasib offered significant benefits compared to the standard intravenous treatment.
BREAST CANCER
Immune check-point inhibitors (ICIs) are monoclonal antibodies, used for immunotherapy in the oncology field. ICIs block proteins that stop the immune system from attacking the cancer cells. Most known class representatives are PD-1 inhibitors and PD-L1 inhibitors. ICIs have been approved to treat a variety of cancers and many ICIs are under development. The WHO Essential Medicines List (EML) committee recognised their importance as a therapeutic class. Some of them are listed on the EML for the treatment of skin melanoma. In several cases, the WHO EML Committee recommended to continue working on strategies to improve the affordability of these medicines and also suggested some for consideration for licensing by MPP. Moreover, MPP technology transfer support could support a successful implementation by reducing development time and costs of biosimilar versions for use in LMICs. MPP has prioritized ICIs as a class, and specific molecules, such as pembrolizumab, are listed here as examples of this effort.
Abemaciclib is an oral Cyclin-dependent kinase 4 and 6 (CDK 4/6) inhibitor, approved by the Food and Drug Administration for the treatment of HR+/HER2- advanced breast cancer, recommended as the preferred option in the treatment of advanced breast cancer. CDK 4/6 inhibitors are the recommended preferred option in the treatment of advanced breast cancer. The Essential Medicines List expert committee recognised its potential for future inclusion and recommended to MPP to explore licensing opportunities. Abemaciclib has a similar safety profile to ribociclib but with a different dosing regimen, making it an alternative of interest.
Ribociclib is an oral Cyclin-Dependent Kinase 4 and 6 (CDK 4/6) inhibitor, approved by the Food and Drug Administration for the treatment of HR+/HER2- advanced breast cancer. The Essential Medicines List expert committee recognised its potential for future inclusion and recommended to MPP to explore licensing opportunities.
Trastuzumab subcutaneous is a monoclonal antibody approved by the FDA in 2019 for the treatment of HER2+ overexpressing breast cancer. It is the same monoclonal antibody as intravenous trastuzumab with the advantage of being easier and more rapid to administer.
The intravenous formulation of paclitaxel was added to the EML in 2011 and used in the treatment protocols for many cancers. Pending confirmation of its safety and efficacy, this new mode of oral administration, currently under development, could be particularly promising for LMIC settings, and therefore have been included in the watchlist.
CHRONIC LYMPHOCYTIC LEUKAEMIA
Ibrutinib s a Bruton's Tyrosine Kinase Inhibitor (BTKi) approved by the Food and Drug Administration in 2013 and added to the complimentary list of the Essential Medicines List for the treatment of chronic lymphocytic leukaemia (CLL). Ibrutinib demonstrated major benefits compared to chemo-immunotherapy. The Essential Medicines List expert committee recommended to MPP to explore licensing opportunities.
Zanubrutinib is a Bruton’s Tyrosine Kinase Inhibitor (BTKi) approved by the Food and Drug Administration in 2023 for the treatment of chronic lymphocytic leukaemia (CLL). Recognizing the emerging important role of BTKi as a therapeutic class in the treatment of CLL, the EML Committee advised that it would consider an application for zanubrutinib as therapeutic alternative for inclusion and recommended to MPP to explore it for licensing.
PROSTATE CANCER
Apalutamide is a 2nd generation androgen receptor antagonist approved by the Food and Drug Association (FDA) in 2018. As a class of drugs, 2nd generation androgen receptor antagonists improve overall survival in prostate cancer patients. Apalutamide is a strong potential alternative candidate.
Darolutamide is a 2nd generation androgen receptor antagonist approved by the Food and Drug Association in 2019. As a class of drugs, 2nd androgen receptor antagonists improve overall survival in prostate cancer patients. Darolutamide is a strong potential alternative candidate.
The intravenous formulation of paclitaxel was added to the EML in 2011 and used in the treatment protocols for many cancers. Pending confirmation of its safety and efficacy, this new mode of oral administration, currently under development, could be particularly promising for LMIC settings, and therefore have been included in the watchlist.
Diabetes
Currently, 537 million adults (10.5% of the global population) are grappling with diabetes. Projections indicate that this number will climb to 643 million by 20301. Approximately 240 million individuals worldwide live with undiagnosed diabetes, meaning nearly one out of every two adults is oblivious to their condition. Notably, 90% of these undiagnosed cases are concentrated in low- and middle-income countries (LMICs). Type 1 diabetes afflicts over 1.2 million children and adolescents, with 54% of them under the age of 15. Type 2 diabetes is the most common type of diabetes, accounting for over 90% of all diabetes worldwide. Globally, the prevalence of type 2 diabetes is high and rising across all regions and has also become a concern in children and young people as a result of an increasing prevalence of obesity.
Cardiovascular diseases
Cardiovascular diseases (CVDs) stand as the leading global cause of death, accounting for 32% of all deaths in 2019, with an estimated 17.9 million lives lost. Over three-quarters of CVD deaths occur in low- and middle-income countries. These diseases were responsible for 38% of the 17 million premature deaths (those under 70 years old) attributed to noncommunicable diseases in 2019. Early detection of cardiovascular disease is paramount to initiate timely management through counselling and medication2. Despite high-quality scientific evidence of the benefits of different classes of drugs in preventing and controlling cardiovascular disease, their current use remains low.
1 International Diabetes Federation - Diabetes Atlas 2021 (last accessed 25 Sept. 2023).
2 World Health Organization - Cardiovascular diseases Fact Sheet.
DIABETES
The incretin hormones are gut hormones that amplify nutrient-induced insulin secretion in response to meal intake. Incretin peptides, principally Glucagon-like Peptide-1 (GLP-1) and Gastric inhibitory polypeptide (GIP), regulate islet hormone secretion, glucose concentrations, lipid metabolism, gut motility, appetite and body weight, and immune function, providing a scientific basis for utilizing incretin-based therapies in the treatment of type 2 diabetes1. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are attractive options for the treatment of T2DM as they effectively reduce glycaemia and weight while posing a low risk of hypoglycaemia. Some GLP-1 RAs also have documented beneficial effects on the cardiovascular system, chronic kidney disease and non-alcoholic fatty liver disease.
1 Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013.
CARDIOVASCULAR & METABOLIC DISORDERS
Cardiovascular fixed-dose combinations of several drugs (commonly known as “polypills”) have been shown to rationalize treatment regimens, improve adherence and better control risk factors. Polypills for the prevention of cardiovascular disease are made up of cholesterol-lowering agents, one or more blood pressure-lowering agents and, if necessary, acetylsalicylic acid (aspirin). Their recent inclusion in the WHO Essential Medicines List should improve the accessibility and affordability of these essential medicines, which have the potential to save millions of lives every year.
The incretin hormones are gut hormones that amplify nutrient-induced insulin secretion in response to meal intake. Incretin peptides, principally Glucagon-like Peptide-1 (GLP-1) and Gastric inhibitory polypeptide (GIP), regulate islet hormone secretion, glucose concentrations, lipid metabolism, gut motility, appetite and body weight, and immune function, providing a scientific basis for utilizing incretin-based therapies in the treatment of type 2 diabetes1. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are attractive options for the treatment of T2DM as they effectively reduce glycaemia and weight while posing a low risk of hypoglycaemia. Some GLP-1 RAs also have documented beneficial effects on the cardiovascular system, chronic kidney disease and non-alcoholic fatty liver disease.
1 Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013.
On 11 April 2024, on the occasion of the International Day for Maternal Health and Rights, MPP announced the signing of a memorandum of understanding (MoU) with Ferring Pharmaceuticals that includes a conditional licence agreement for heat-stable carbetocin (HSC). This innovative drug is vital for preventing post-partum haemorrhage, a serious condition that leads to the death of approximately 66,500 women annually in low- and middle-income countries. HSC, which has been recognised as essential by the EML core list since 2019, matches oxytocin in effectiveness and safety, and crucially, does not require refrigeration. This is particularly beneficial in regions with limited resources.
Currently, we are not specifically prioritising medicines within the maternal health area. However, it is important to note that our prioritization process is dynamic and conducted on a rolling basis. New candidates for in-licensing could be considered and added to our priority or watch lists at any time, should they meet our framework criteria.
Non-communicable diseases (NCDs) are the leading cause of death among women, accounting for three-quarters of female deaths each year. Breast and cervical cancers are the most diagnosed and deadly cancers for women globally, with a significant number of these deaths occurring in underprivileged areas. To address these challenges, MPP is focusing on specific treatments, including the immune checkpoint inhibitor class and five other products that are key in combating breast and cervical cancers. This targeted approach is part of MPP’s commitment to improving healthcare outcomes for women around the world.
World Health Organization - Maternal health Fact Sheet (last accessed 25 Sept. 2023).
World Health Organization - Maternal mortality Fact Sheet (last accessed 25 Sept. 2023).
World Health Organization - A roadmap to combat postpartum haemorrhage between 2023 and 2030.
Across most disease areas in which MPP has worked, access to medicines for children lags behind that for adults, and in many cases important medicines are not available in formulations that can be taken easily by young children.
Since its inception, MPP has prioritised working with manufacturers on bringing needed paediatric formulations to market, including accelerating their development and facilitating uptake. This contribution has increasingly taken place in partnership with other key stakeholders in the paediatric field.
Moving forward, MPP will continue to place particular focus on addressing the needs of children across all disease areas in which it operates. Its contribution in paediatrics will be framed in alignment with the Global Accelerator for Paediatric Formulations Network (GAP-f) a WHO network of which MPP is a founding member, to ensure that the most-needed, optimal paediatric formulations are prioritised, developed, and made available to children in an accelerated manner.
Sickle cell disease
In 2019, sickle cell disease (SCD) resulted in 47,932 deaths1. In 2019, 605,000 individuals were born with SCD, while a total of 5.69 million people were living with the condition2. Notably, sub-Saharan Africa bears the highest SCD prevalence, with approximately 80% of cases occurring in this region3. The mortality rate for children under 5 years of age in this region ranges from 50% to 80%4.
Respiratory Syncytial Virus
Respiratory syncytial virus (RSV) is a virus that causes acute respiratory infection in individuals of all age groups. While most infants and young children experience mild, cold-like symptoms, some infants, especially with their first infection, develop lower respiratory tract disease such as pneumonia and bronchiolitis (swelling of the small airway passages in the lungs), that often leads to an emergency department or physician office visit. Premature infants, and those with chronic lung disease of prematurity or significant congenital heart disease, are at highest risk for severe RSV disease5. RSV caused an estimated 101,400 deaths in children under 5 in 20196.
Cystic fibrosis
Cystic fibrosis, a rare, progressive, life-threatening disease, caused by mutations in the Cystic Fibrosis Transmembrane conductance Regulator (CFTR) gene that results in the formation of thick mucus that builds up in the lungs, digestive tract, and other parts of the body. It leads to severe respiratory and digestive problems as well as other complications such as infections and diabetes. There is no cure and people with Cystic Fibrosis receive daily treatments depending on the severity of their symptoms7. There are no global annual deaths estimates for cystic fibrosis and related life expectancy, but it is generally assumed that in the absence of CFTR modulators, the median life expectancy for cystic fibrosis would be around 24.5 years (compared to 46 in presence of CFTR)8.
1 Institute for Health Metrics and Evaluation - Global Burden of Disease Study 2019.
2 Institute for Health Metrics and Evaluation - Global Burden of Disease Study 2019.
3 Sickle-cell disease. Lancet. 2010.
4 Sickle cell disease and pulmonary hypertension in Africa: a global perspective and review of epidemiology, pathophysiology, and management. Am J Hematol. 2008.
5 World Health Organization - Respiratory Syncytial Virus (RSV) disease (last accessed 25 Sept. 2023).
6 Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. The Lancet. 2022.
7 American Lung Association - Cystic Firbrosis (last accessed 25 Sept. 2023)
8 Worldwide rates of diagnosis and effective treatment for cystic fibrosis. Journal of cystic fibrosis. 2022.
CYSTIC FIBROSIS
The combination of the active substances elexacaftor, ivacaftor and tezacaftor is the first triple combination therapy available to treat patients with the most common cystic fibrosis mutation. It has been approved for patients 12 years and older with cystic fibrosis who have at least one F508del mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which is estimated to represent 90% of the cystic fibrosis population.
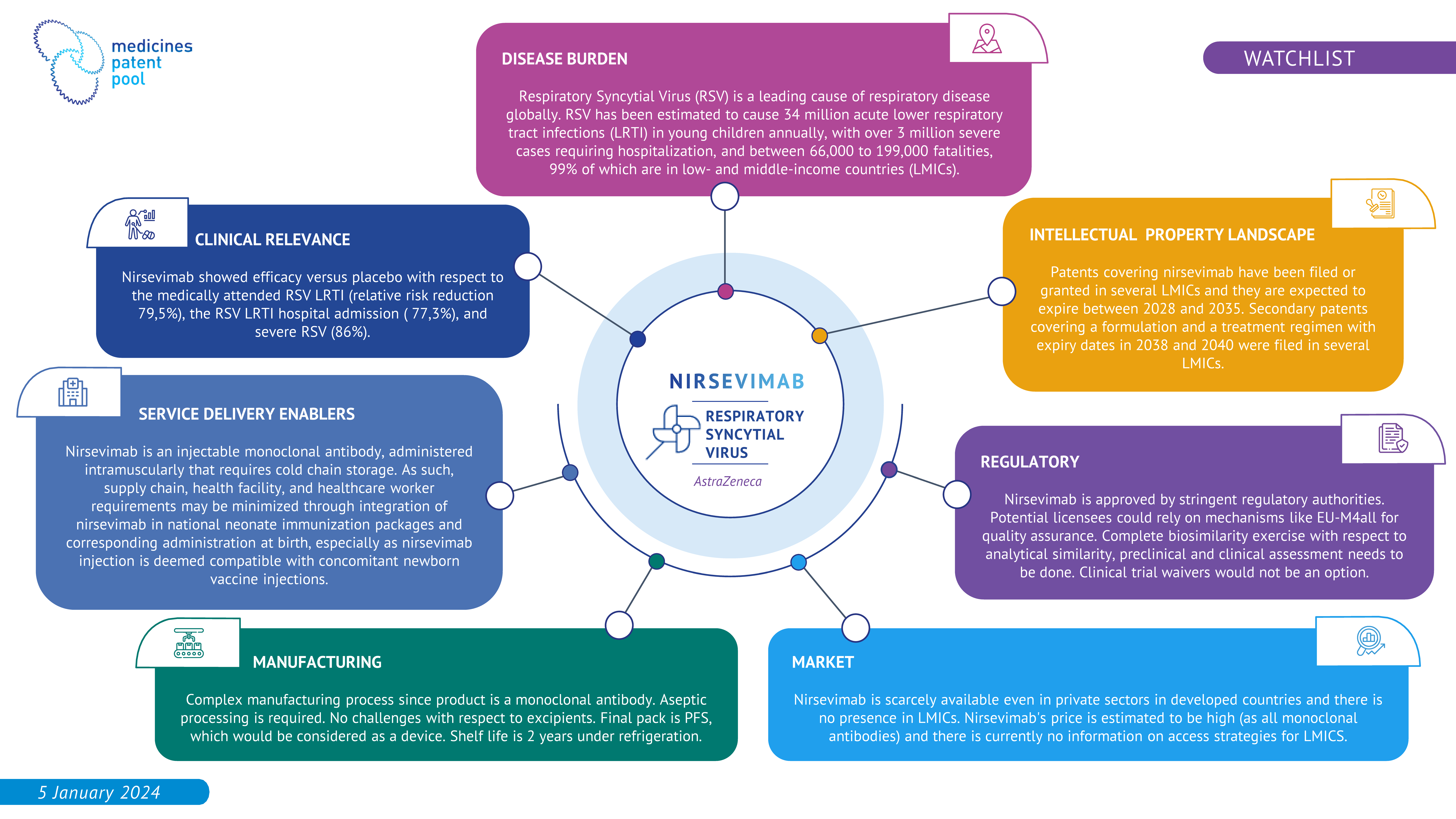
RESPIRATORY SYNCYTIAL VIRUS
Nirsevimab is a monoclonal antibody with activity against Respiratory Syncytial Virus (RSV). Monoclonal antibodies are laboratory-made proteins that mimic the immune system’s ability to fight off harmful pathogens such as viruses. One dose of nirsevimab administered to infants as a single intramuscular injection prior to, or during RSV season, may provide protection during the RSV season. Infants have the highest incidence of severe disease, peaking at 1–3 months of age.
Clesrovimab is a monoclonal antibody with activity against Respiratory Syncytial Virus (RSV).
Monoclonal antibodies are laboratory-made proteins that mimic the immune system’s ability to fight off harmful pathogens such as viruses.
Although the product is still in development, one dose of clesrovimab administered to infants as a single intramuscular injection may provide protection to RSV. Infants have the highest incidence of severe disease, peaking at 1–3 months of age.
SICKLE CELL DISEASE
Voxelotor is a haemoglobin oxygen-affinity modulator approved by the FDA in 2019 for the treatment of sickle cell disease. About 90% of the global sickle cell population lives in LMICs. Voxelotor has a different mode of action from that of conventional standard of care therapies, which makes it relevant as additional treatment or in case of intolerance.
MPP’s initial work began with infectious diseases including HIV, viral hepatitis and tuberculosis (TB), and obtained licences that resulted in high public health impact. In 2018, MPP’s mandate expanded to target patented medicines included in the WHO Model List of Essential Medicines or with potential for future inclusion, which encompass a whole range of disease areas, including cancers, diabetes and cardiovascular diseases, and is exploring other areas of intervention, as relevant. Additionally, MPP has been working on COVID-19 interventions since 2020. MPP’s new strategy for the 2023-2025 period embraces a disease agnostic approach, by which patented medicines for which an MPP intervention would potentially make a difference in public health, might be considered for prioritisation, regardless of the health area.
MPP’s work started with small molecules, and after conducting a feasibility study on expanding access to biotherapeutics in 2022, expanded its mandate on biologics. Moreover, given their ground-breaking potential impact, long-acting technologies and formulations designed to achieve longer exposure to medicines are considered for prioritisation since 2021, together with any relevant novel medical technologies for which an MPP intervention might generate positive impact for public health.
In line with MPP new strategy, candidate products in earlier stages of development are increasingly being considered for prioritisation. Therefore, MPP might consider for prioritisation candidates at any stage of development, from pre-clinical to marketed.
To read previous priority reports, follow this link
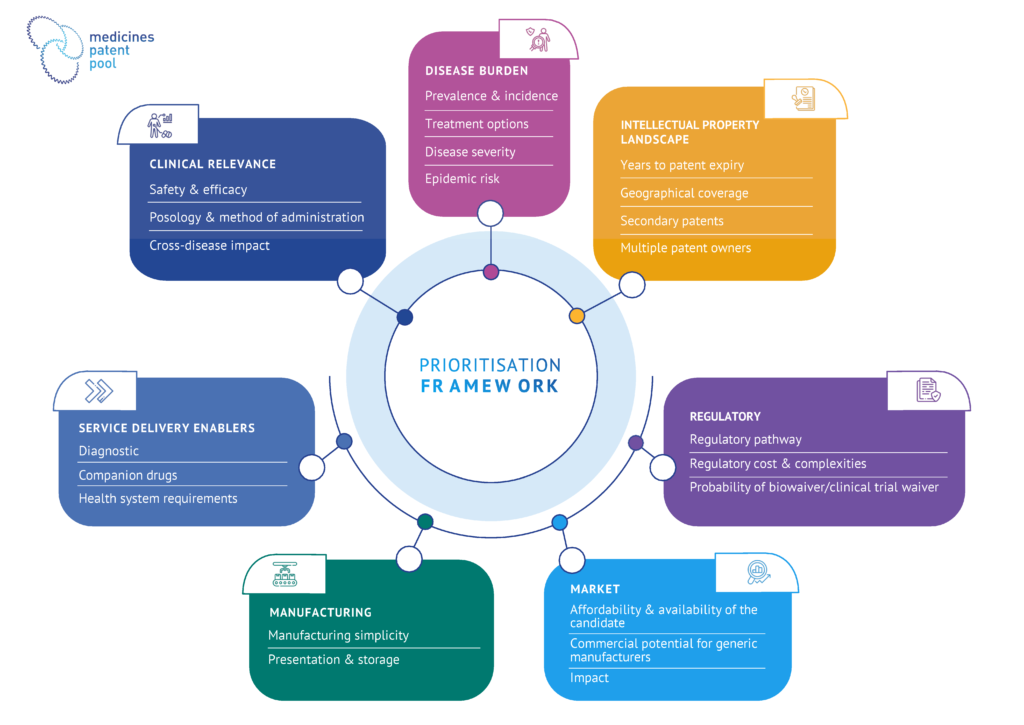
The MPP prioritisation framework was designed to answer the following three questions, as guiding principles. By addressing these questions, MPP collects insights about public health and access dimensions of the products assessed, as well as insights to assess the potential impact of an MPP intervention.
- Does the product address a public health need?
This question is assessed through the first pillar of the framework: the public health pillar, where the burden of the health condition is assessed, as well as the advantages of the candidate product over existing alternatives of care for this condition.
- Are there any access hurdles (anticipated or existing) for the product in LMICs?
This question is assessed through the second pillar: the access pillar. It includes access considerations on which MPP directly intervenes (e.g. intellectual property), as well as additional access considerations which may be important in the treatment cascade (e.g. access to diagnostics).
- Would MPP intervention bring improvement over the status quo?
This question elaborates on both public health and access pillars, and it ensures that candidate products are prioritized where an MPP intervention could yield the greatest impact.
The assessment of medicines for licensing is carried out in collaboration with MPP’s Scientific Advisory Panel and Community Advisory Panel.
Medicines for which expanded access could provide significant health benefits over standards of care, and where voluntary licensing through MPP would lead to substantial public health impact.
Medicines for which expanded access could provide significant health benefits but for which supporting data are lacking and/or key challenges need to be addressed for expanded access through MPP licensing to provide significant benefits and lead to substantial public health impact. Additionally, we include medicines in the watchlist when a potential added benefit might be obtained through an MPP licence, but where a full assessment is still ongoing.
If you would like to know more about MPP, its work on medicines prioritisation or want to collaborate, please contact science@medicinespatentpool.org





 ALL HEALTH AREAS
ALL HEALTH AREAS