A closer look: TLD
TLD is a generic HIV combination available in low- and middle-income countries
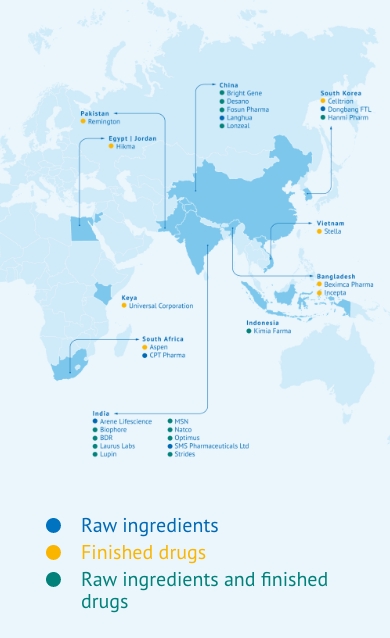
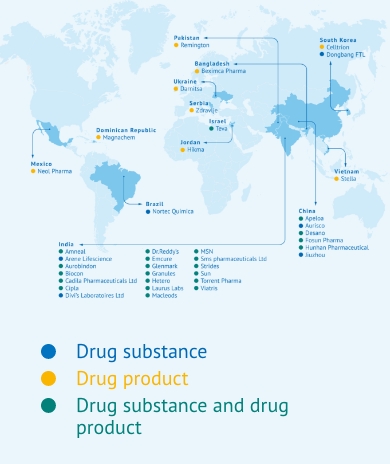
By 2021 the fixed-dose combination of TLD in a single dosage – developed by MPP’s manufacturing partners – was already reaching almost 20 million people. The number of low- and middle-income countries receiving the WHO-preferred TLD treatment for HIV passed the milestone of 100 countries in 2022. Eleven of MPP’s generic manufacturing licensees have now supplied more than 726 million packs of TLD, which means that it is now the most widely used HIV regimen in the world.